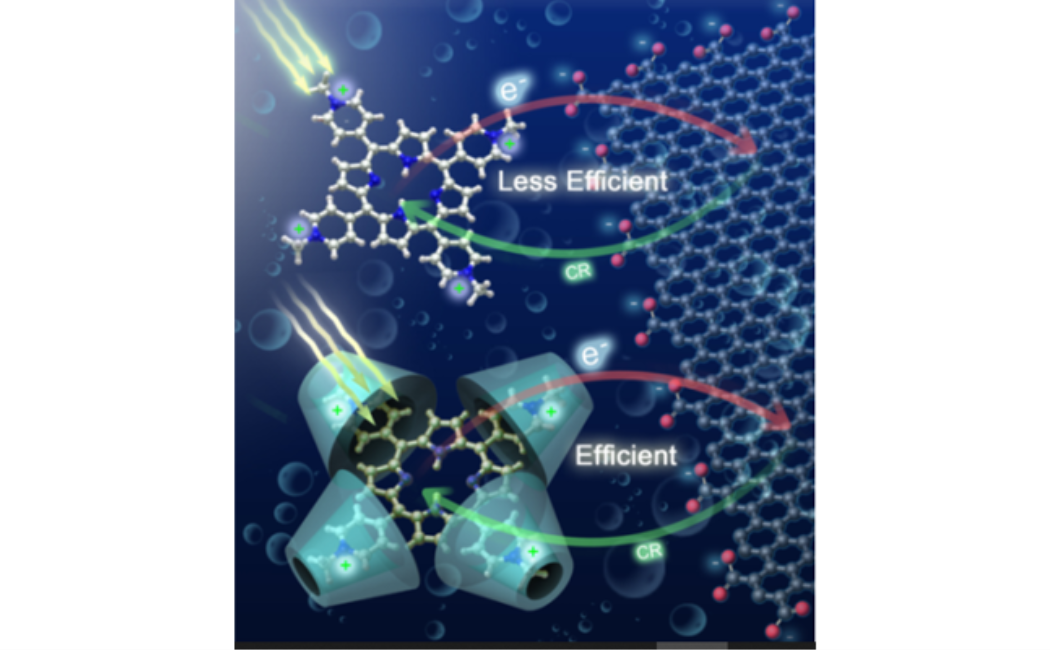
The discovery of graphene was a breakthrough in the search for new materials for future electronic and energy applications. In addition, its high-quality two-dimensional (2D) crystal lattice has provided graphene with remarkably unique electronic properties and high electrical conductivity. On the other hand, the porphyrin family of compounds has been central to studies of organic solar cells due to their remarkable photo-electrochemical properties, and they have recently received very special interest in the photovoltaics community. They are among the most promising components of new materials for future electronic devices. Based on this, we extended our interest to the charge transfer of graphene (GC)-porphyrin interfaces not only to control the rate of the process, but more importantly to improve the electron injection efficiency at the graphene interface. Here, we carefully selected three porphyrin structures with different charge localizations of the meso unit and different redox properties of the porphyrin cavity (Figure 1, left panel) to understand the ultrafast electron injection event at the GC–porphyrin interface from the molecular structure point of view (Figure 1, left panel).
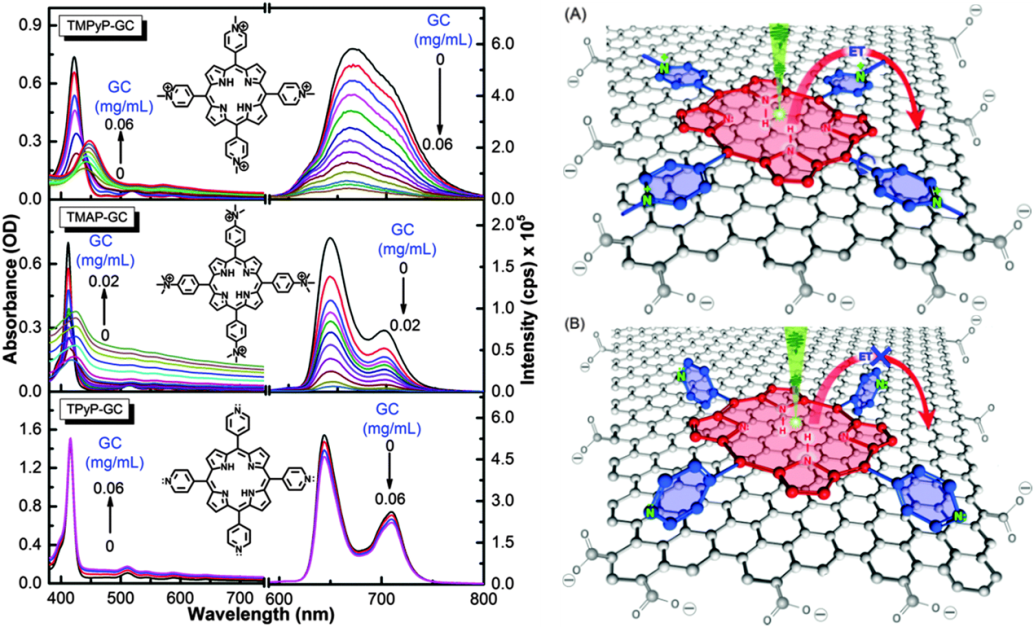
Figure 1: (left panel) Absorption (left) and fluorescence (λex= 515 nm) (right) of the investigated porphyrins recorded upon the addition of GC (concentrations in mg/mL as indicated in the figure). (Right panel) the conformational changes of the porphyrins upon addition of GC: TMPyP (A) and TPyP (B).
Our results clearly demonstrate the possibility of modulating the CT rate between porphyrin and the GC surface by controlling both the distance and electronic coupling between the donor and the acceptor units. This can be achieved by changing the electronic structure of the meso unit and the redox properties of the porphyrin macrocycle. Our results show that the CT process between porphyrins and GC can be tuned from zero to very sufficient and ultrafast (see Figure 1). More specifically, the results demonstrate that only positively charged porphyrin can approach closer after molecular flattening on the GC surface for ultrafast electron injection as inferred from the formation of the cationic species of TMPyP.+ and TAMP. In addition, controlling the efficiency of the interfacial charge-transfer process is a research direction that has not yet seen much progress. In this communication, we used β-Cyclodextrin (β-CD) as an external cage to block intramolecular charge transfer (ICT) between the macrocycle and its positively charged meso units of 5,10,15,20-tetra(1-methyl-4-pyridino)porphyrin (TMPyP) (Figure 2 left panel). Subsequently, the electron density is localized on the cavity of TMPyP and its oxidation potential is reduced, significantly increasing the availability of donating electrons to the electron acceptor component. Next, we explored the interaction of the resulting configuration (TMPyP-β-CD) with graphene carboxylate (GC) as an electron-acceptor unit in aqueous solution after 650 nm of photoexcitation. We found that the electron injection efficiency from TMPyP to GC in the presence of β-CD is 120% higher than without β-CD (Figure 2, left panel). The work presented here will advance our understanding of GC interfaces for electronic and solar energy applications.
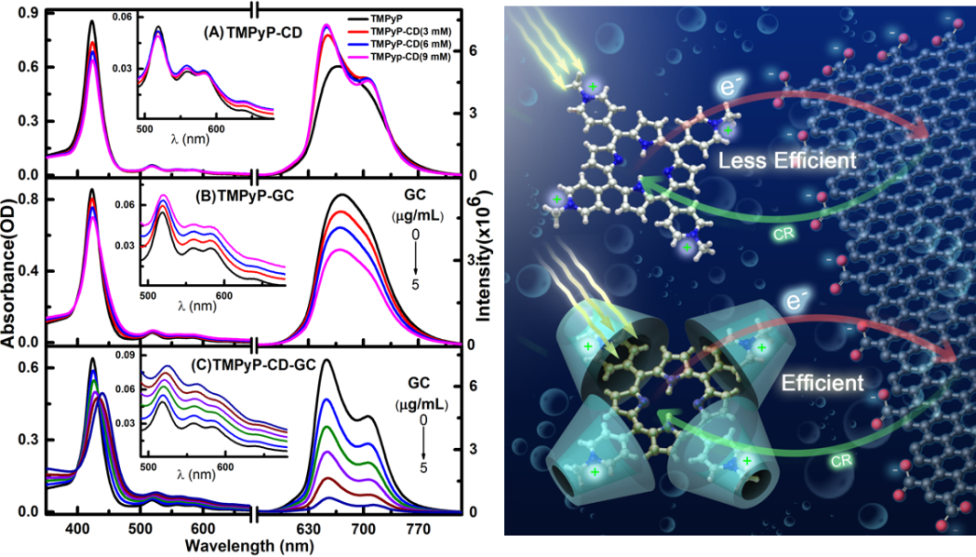
Figure 2: (Left Panel) Absorption (left) and fluorescence (λex = 580 nm) (right) of TMPyP in the presence of CD (A), GC (B), and CD-GC (C). Right Panel, A schematic diagram of electron transport at the porphyrins-GC interface with and without CD.